Citi Risks Associated With Genetic Research Are Best Described as
Digital identity has evolved from centralized to federated models. Decide on data collection approaches including research design methodology and tools 4.

Etiology Etiology Is The Identification Of Causes Of A Disease And The Relevant Risk Factors Associated With That Disease T Obesity Risk Factors Endocrine
Next we investigated how the genetics of risk taking relates to the genetics of other health-related traits.

. And 4 willing to participate. Topics include assessing risks balancing risks and potential benefits minimizing and managing risks certificates of confidentiality and ways to address risks in the informed consent document and process. These involve medical psychosocial and economic risks such as the possible loss of confidentiality of insurability and employability a change in immigration status potential limits on education options and social stigmas.
It provides personalized information about your health disease risk and other traits. All IFR subjects met the following criteria. The involvement of vulnerable populations as research subjects.
To evaluate the association between individual genotypes and the safety and efficacy of a particular drug or class of drugs Investigator A has biological specimens that are coded and linked to identifiers of the source individuals. And 4 willing to participate. Evaluate risks to subjects protection against these risks potential benefits of the research and the importance of the knowledge to be gained.
3 fluent in English. Genetic research also may include the construction of pedigrees maps of the distribution of a particular trait or condition among related individuals or family medical histories. While physical risks are minimal in social and behavioral science research risks associated with participation in social and behavioral science research are often more elusive and less predictable.
Level of risk to subjects associated with the project. There is also research suggesting that immune function and personality are linked. When disclosure is to be attempted over the patients refusal the burden should be on the person.
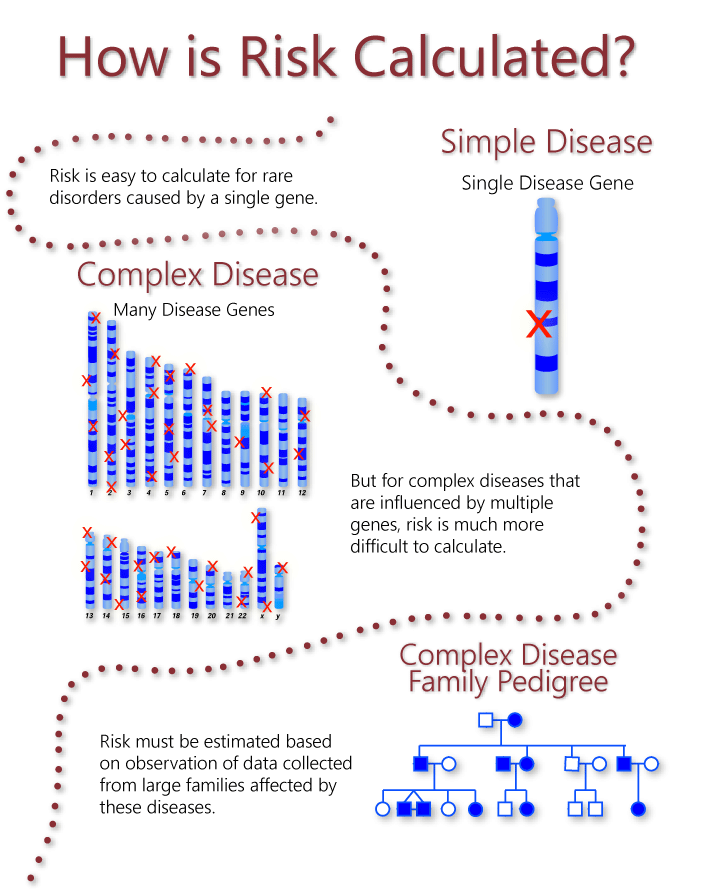
Direct-to-consumer genetic testing promotes awareness of genetic diseases. Genetic research studies may present unique risks to human subjects and their relatives. Among the risks typically associated with genetic research investigators IRBs and research subject advocates among others have identified the potential adverse impact on insurability or employability if genetic information about the subject obtained as part of the research was disclosed to or sought by insurers or employers.
All of the above. For service providers this solution is neither secure nor efficient. Every interaction in a research context is a communication of some sort and communications can go awry.
The Human Subjects Research HSR course includes topics such as the basics of IRB regulations and the review process assessing risk to participants genetic research informed consent research with vulnerable subjects and other relevant issues 2. All IFR subjects met the following criteria. 1 self-described as high risk for developing cancer due to a familial history of hereditary cancer as determined by answering yes to have you or an immediate family member ever had a blood test to identify if there was a mutation.
Describes why workersemployees may be a vulnerable population when they participate in research and the potential risks and benefits associated with research involving workersemployees. Investigators who are not affiliated with the UI who are engaged in a UI research study and whose IRB of record will be UI IRB-01 or UI IRB-02 as designated by a formal written agreement. Determine research objective key questions and audiences 2.
Develop a data analysis plan 5. An investigator at another institution is considered to be engaged in research as described in guidance from the Office of Human Research Protections. 1 self-described as high risk for developing cancer due to a familial history of hereditary cancer as determined by answering yes to have you or an immediate family member ever had a blood test to identify if there was a mutation.
Beyond federated identity a new architecture of decentralized identity is emerging. A lot has changed in human subject research since 1991. You know things like the widespread use of electronic systems in research genetic research and its associated risks different ways of obtaining informed consent the rise of central IRB reviews of research etc.
The physical risks associated with most genetic tests are very small particularly for those tests that require only a blood sample or buccal smear a method that samples cells from the inside surface of the cheek. The procedures used for prenatal diagnostic testing called amniocentesis and chorionic villus sampling carry a small but real risk of losing the pregnancy. 3 fluent in English.
Direct-to-consumer genetic testing has both benefits and limitations although they are somewhat different than those of genetic testing ordered by a healthcare provider. 2 between 1865 years of age. Unlike biomedical clinical trials risks associated with social and behavioral science research are often elusive and less predictable.
The Collaborative Institutional Training Initiative CITI Program is dedicated to promoting the publics trust in the research enterprise by providing high quality peer-reviewed web-based educational courses in research ethics compliance and professional development education. Federated identity only addresses authenticationevery other aspect of identity is still based on the centralized model. It also discusses protections that need to be afforded to workersemployees.
Confidentiality should be breached and relatives informed about genetic risks only when 1 attempts to elicit voluntary disclosure fail 2 there is a high probability of irreversible harm that the disclosure will prevent and 3 there is no other reasonable way to avert the harm. CITI EDUCATION Email was sent to Listserv on 11321 as a courtesy reminder that study teams need to monitor and ensure that CITI education remains up to date Policy went into effect 12018 with 3 year expiration Many research staff have training expiring within the next 3. Although gene transfer is another form of genetic research this guidance document does not apply to gene transfer research.
Identify information sources to address key research questions 3. A systematic investigation designed to develop or contribute to generalizable knowledge may include. 2 between 1865 years of age.
Research with human subjects 1 2. We found that risk taking shares a genetic basis with aspects of body composition such as childhood obesity and waist-to-hip ratio. Develop a dissemination plan to share the research findings 6.
The type of research being conducted eg an educational intervention a survey an ethnographic observation etc The sensitivity of the research questions or complexity of the research design. Which choice best describes the purpose of most pharmacogenomic research.

Genetics Problems Worksheet Answer Key Awesome 14 Best Of Pedigree Worksheet With Answer K Genetics Practice Problems Word Problem Worksheets Biology Worksheet

Faq About Genetic Testing Genetic Testing Nutrigenomics Genetics Facts
No comments for "Citi Risks Associated With Genetic Research Are Best Described as"
Post a Comment